Four Parameters Used to Describe the Behavior of a Gas
What are the Factors that Affect the Behavior of Gases. Their properties are easy to study.
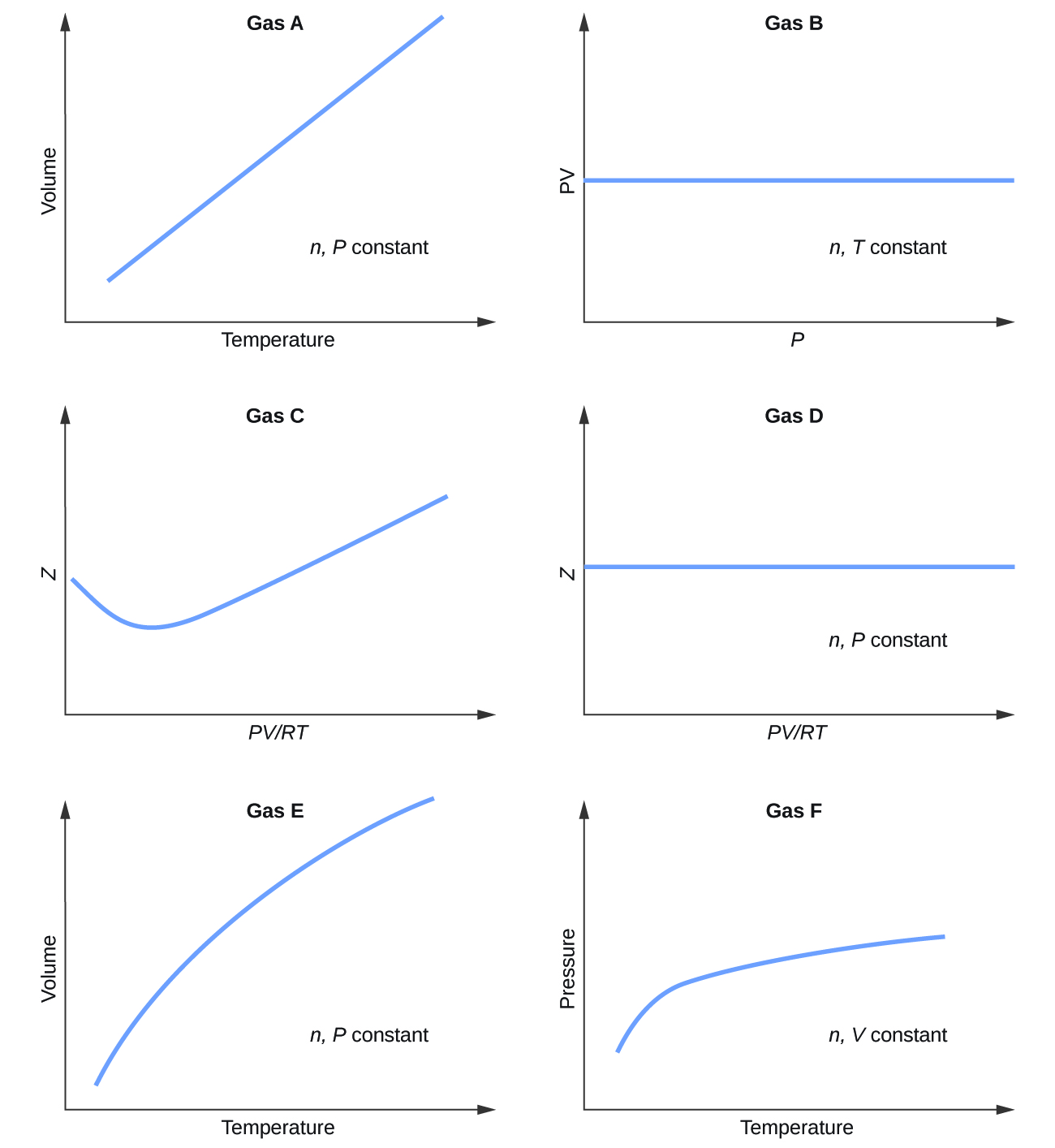
9 6 Non Ideal Gas Behavior Chemistry
Ideal gases are also known as a perfect gas.
. Learn vocabulary terms and more with flashcards games and other study tools. As the temperature increases the volume of the gas also increases due to an expansion of gas molecules. We can explore the relationship between the liter and cubic meter by examining the following conversion factors.
Solid liquid gas plasma and the Bose-Einstein condensate. Get Best Price Guarantee 30 Extra Discount. P V nb nRT P V n b n R T.
Temperature is always in Kelvin. Amount of gas n. Foundations of College Chemistry 5th Edition Edit edition Solutions for Chapter 13 Problem 14E.
Transcribed image text. Solved Expert Answer to What are the four parameters used to describe the behavior of a gas. The van der Waals equation modifies the ideal gas law to correct for this excluded volume and is written as follows.
T 0 0 C 273 0 K. Amount of gas n. Here p is the pressure v is the volume per mole or molar volume R is the universal gas constant and T is the absolute thermodynamic temperature.
We have 113 mL of gas with a mass of 1130171 g. Pressure P volume V number of moles n and temperature T. Following are the factors that affect the behaviour of gases.
Four variables are used to describe the condition of a gas. Since the kinetic energy is mv 2 the same molecule will increase in velocity as temperature increases. And were told the pressure a 7.
It is a combination of several different laws that describe the behavior of gases. These 4 variables are in a delicate balance and if one of the variables is changed one or more of the variables may change relieve the stress on the gas system. So what were gonna do is use the ideal gas equation and solve for n which is the number of moles and then well use fat number and this mass that were given to.
In a closed system the volume of the gas is the same as the volume of the container. Download Table Parameters Used to Describe Viscous Flow Behavior from publication. In this approximation the gas molecules are considered hard spheres.
Pressure P volume V temperature T and amount n in moles. What are the four parameters used to describe the behavior of a gas. Factors Affecting Gas Pressure pages 414417 4.
We will examine separately how the volume temperature and amount of gas each affect the pressure of an enclosed gas sample. It establishes a relationship among the four different gas variables such as pressure P VolumeV TemperatureT and amount of gas n. AT ST or NT.
Out of these gases are a special state. Review questionsWhat are the four parameters used to describe the behavior of a gas. Standard Temperature and Pressure STP or Standard Conditions SC.
The SI unit of volume the cubic meter m 3 has magnitude that is a bit too large in consideration of the amounts and ambient conditions under which gases are normally studied. Need your ASSIGNMENT done. Start studying Gen Chem Exam 4.
What are the four parameters used to describe the behavior of a gas. Gas Law Simulation Write-up There are 4 gas variables that are used to describe the behavior of gases. Molar mass Number of Moles Temperature Density Volume Pressure Molar mass Number of Moles Temperature Density Volume Pressure.
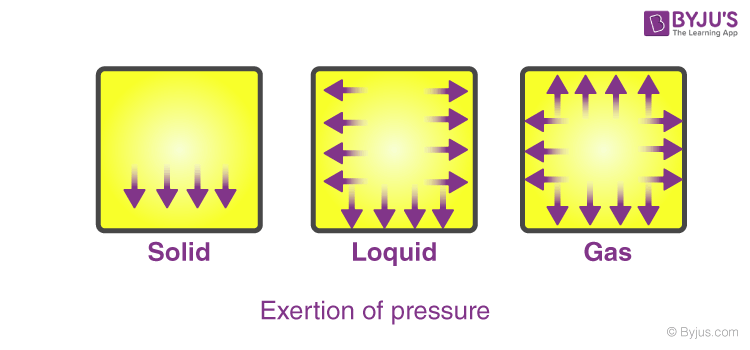
There are 5 main states of matter. Temperature is related to the kinetic energy of the gas and is measured in Kelvin K. 21 millimeters of mercury and the temperature is 32 degrees Celsius and were asked to find the Moeller method of the guests.
These laws tell us about the behavior of gases. College Chemistry 3rd Edition Edit edition Solutions for Chapter 12 Problem 4QC. Which variable is NOT used to describe the amounts and properties of gases.
Viscosity in the System AnorthiteAlbite Viscosities of several compositions in the albite-anorthite system. What are the four parameters used to describe the behavior of a gas. Where k Boltzman constant and N number of gas molecules.
Combined these form the Ideal Gas Law equation. Temperature T Volume V Pressure P Quantity n The above-mentioned factors are inter-related and they are given as follows. P 1 atm or its equivalents.
To a rough degree the. Use our paper writing service to score better and meet your deadline. Typically measured in moles.
Due to this distance colorless gases are invisible to the human eye and are studied using four measurable parameters. Get solutions Get solutions Get solutions done loading Looking for the textbook. The ideal gas law is a mathematical equation that relates all of these parameters.
At absolute zero 0 K molecules stop moving entirely the gas is as cold as anything can get. The volume of gas in a sample is measured in liters L. They are pressure volume temperature and the amount of the gas as measured by the number moles.
List the name the symbol and a common unit for the four variables that are generally used to describe the characteristics of a. We see that gases follow certain laws known as the gas laws. Get solutions Get solutions Get solutions done loading Looking for the textbook.
The available volume is now represented as V nb V n b where b is a constant that is specific to each gas. This expression is called the ideal or perfect gas equation of state since all real gases show small deviations from it although these deviations become less significant as the density is decreased. We can also use an equivalent equation given below.
Assuming ideal gas behavior for which of the following gases does PVnT R 008206 L atmmol K. The universal gas constant R is a number that satisfies the proportionalities of the pressure-volume-temperature relationship. Review Question 1211 What are the four parameters used to describe the behavior of a gas.
1 atm 1 atmosphere 760 torr 760 mm 76 m Hg. P is the pressure V is the volume N is the number of moles of gas R is the universal gas constant and T is the absolute temperature. Click Here to Make an Order Click Here to Hire a Writer.

What Are The Properties Of Gases Physical Properties Of Gases

No comments for "Four Parameters Used to Describe the Behavior of a Gas"
Post a Comment